

This page is shared under a CK-12 license and was authored, remixed, and/or curated by Melissa Alviar-Agnew, Henry Agnew, and Lance S. If a balanced atom gains one or more electrons, it will become a negatively charged anion. If a balanced atom loses one or more electrons, it will become a positively charged cation. Anions are ions that are negatively charged. The atomic mass unit (amu) is a unit of mass equal to one-twelfth the mass of a carbon-12 atom Cations are ions that are positively charged.As a result, a neutral atom must have an equal number of protons and electrons. The positive charge on a proton is equal in magnitude to the negative charge on an electron.Protons and neutrons have approximately the same mass, but they are both much more massive than electrons (approximately 2,000 times as massive as an electron).Like protons, neutrons are bound into the atom's nucleus as a result of the strong nuclear force. Study with Quizlet and memorize flashcards containing terms like A positive ion has more a) electrons than neutrons b) electrons than protons c) protons than neutrons d) protons than electrons e) neutrons than protons, 2 protons attract each other gravitationally and repel each other electrically. With an electron removed, the atom possesses.

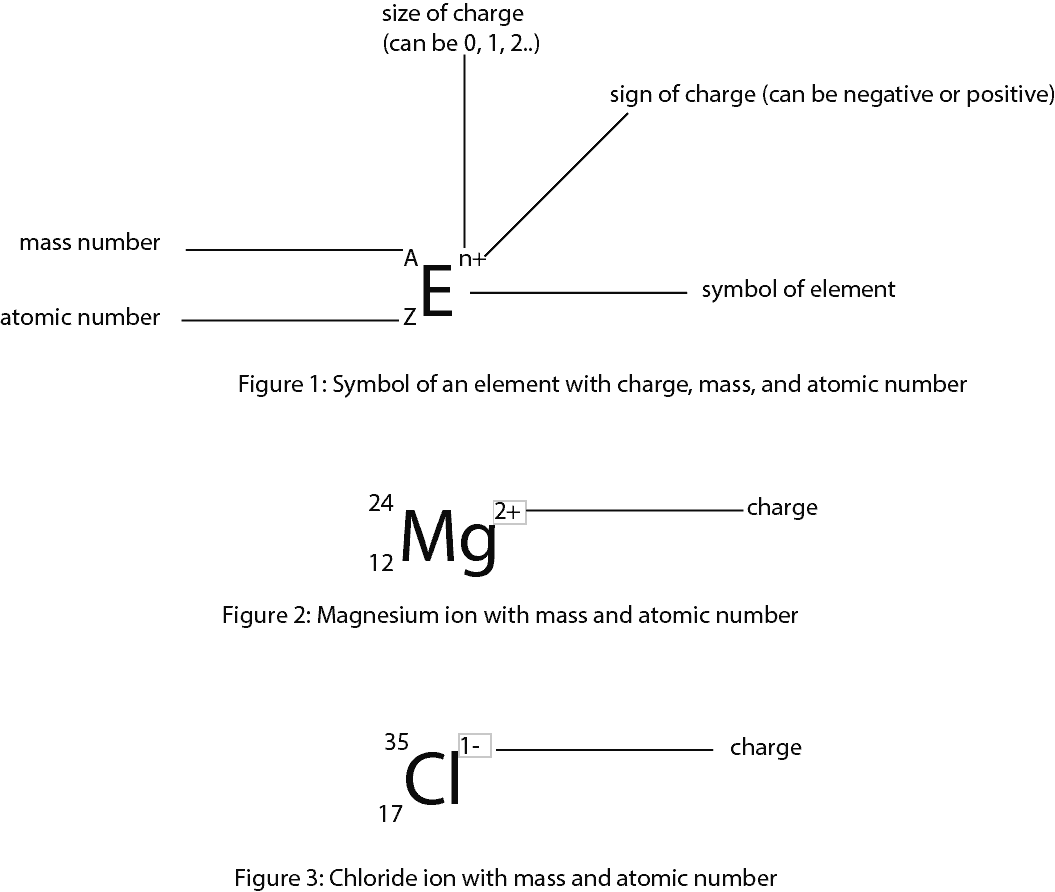
Protons are bound together in an atom's nucleus as a result of the strong nuclear force. Protons are a type of subatomic particle with a positive charge.Electrons are a type of subatomic particle with a negative charge.\): Properties of Subatomic Particles Particle


 0 kommentar(er)
0 kommentar(er)
